NeurAxis, Inc. (NYSE: NRXS) is a biotech company developing Neuromodulation Therapies to address chronic and debilitating conditions in kids.
The company’s premier product is the proprietary Percutaneous Electrical Nerve Field Stimulation (PENFS) technology. The technology already has one FDA indication, with ongoing clinical trials in multiple pediatric conditions. The focus is on the unfulfilled healthcare needs of children.
History and Mission
NeurAxis was founded in 2011 and is focused on growing awareness regarding its revolutionary PENFS technology. Its mission is to offer solutions that lead to better patient health outcomes. They believe in improving the quality of life while reducing suffering in pediatric patients. Its IB-Stim therapy has already been approved for functional abdominal pain treatment associated with IBS. The total addressable market for this treatment is 6 million children. NeurAxis has eight patents, with more pending.
Recent Updates
In a recent press release, NeurAxis revealed that studies had shown their IB-Stim technology improved the quality of life for adolescents with IBS. According to the study, patients reported a significant reduction in disability, nausea, abdominal pain, and anxiety after four weeks of treatment (p < 0.05).
The study was conducted at Children’s Hospital of Orange County and involved 31 adolescent patients aged 11 to 18 with functional abdominal pain disorders (FAPDs). Data was collected over four weeks from parents and patients using questionnaires.
According to Brian Carrico, the President and CEO of NeuraAxis, this was the 10th peer-reviewed publication of PENFS technology. He noted that as the body of clinical evidence supporting IB-Stim therapy grew, they actively leveraged the publications to expand written policy coverage. The goal was to drive change that supports IBS-Stim as standard care for FAPDs.
In another study Led by the Cincinnati Children’s Hospital Medical Center, it was concluded that IB-Stim may be a great non-pharmacological alternative for FAPD. According to the press release dated September 26, 2023, it was found that of 101 adolescents who were treated with 4 weeks of IB-Stim, amitriptyline, or cyproheptadine, 59% of patients treated with IB-Stim had failed previous standard medical therapy.
Outcomes were evaluated using validated pediatric questionnaires using Abdominal Pain Index (API), Nausea Severity Scale (NSS), and Functional Disability Inventory (FDI) at baseline and after 3 months. It was found that those who received IB-Stim therapy had seen improvement in abdominal pain (p=0.001) and functional disability (p=0.048). Those treated with amitriptyline had an improvement in abdominal pain (p=0.034).
The study also found that IB-Stim was more effective than cyproheptadine in improving abdominal pain (p=0.04) and did not differ from amitriptyline (p=0.64). Nausea scores were similar across groups (p>0.05).
It was also found that disability scores between groups were only more effective for amitriptyline vs. cyproheptadine (p=0.03). However, they did not differ from amitriptyline compared with IB-Stim (p=0.21).
According to Dr. Adrina Miranda, the Chief Medical Officer of NeurAxis, the comparative evaluation of IB-Stim with standard medical therapy was an exciting development. Dr. Miranda noted that while amitriptyline and cyproheptadine were the most common medications for treating FADP, there was no clinical evidence that they were effective in children. He concluded that it might be that the medications do not perform as well as we think.
NeurAxis Financial Update
According to the company’s Q2 2023 report, the company’s revenue was $646K, a decrease of 5% compared to $682.6K in Q2 2022. They attributed the decline to the ordering patterns of their major customers.
Their gross profits for Q2 2023 were $578.2K, a decline of 4% compared to $603.6K in Q2 2022. Gross margin total 89.5% in Q2 2023, compared to 88.4% in Q2 2022. The increase was attributed to a lower cost of sales. Selling expenses dropped to $78.8K in Q2 2023 compared to $127.4K in Q2 2022 due to lower commission costs, as the commission rate was lowered at the start of 2023.
R&D expenses were $109.8K in the quarter compared to $3.7K in Q2 2022, occasioned by increased spending on product development. For Q2 20223, general and administrative expenses total $1,507.2K, compared to $1,132.1K in Q2 2022. The increase was attributed to a rise in professional fees.
The company recorded a net loss of $2,235.6K in Q2 2023, compared to $1,516.5K in Q2 2022. This represents a net loss of $1.21 per common share compared in Q2 2023 to $0.87 per common share in Q2 2022.
Stock Performance
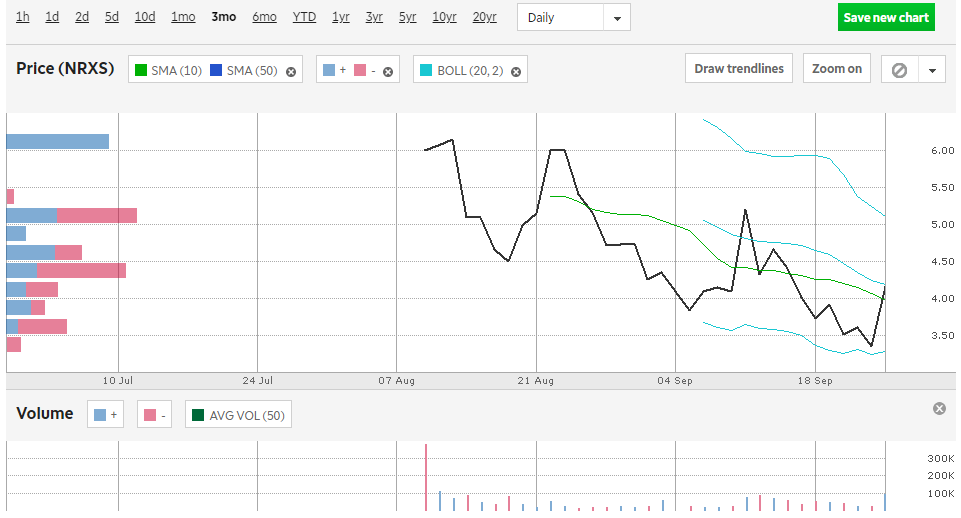
NeurAxis stock is listed on the NYSE, under the ticker $NRXS, and has a price of $3.41 as of September 25, 2023, 9:03 AM ET. The stock has a market cap of $20.44 million, with an average 3-month volume of 52.7K, while the enterprise is valued at $21.85M. Their 1y Target Estimate is $7.50, while the growth estimate for the next year is 89.20%. The stock’s 52-week high was $6.93, while the low was $3.19.
[Click The $NRXS Chart Above To View On Yahoo! Finance]
Final Thoughts
NRXS’s value proposition is solid, and its innovative technology has the potential to impact millions of lives. With recent statements by its CEO indicating widespread approval could be forthcoming, NRXS is a valuable growth stock. The company could become a valuable player in pediatric care. Combining the strengths of its PENFS technology with expert management and the infrastructure to support growth and scale, the company stock’s path of least resistance is growth. They already have a clear pathway to profitability.
Click Here for Updates on $NRXS – It’s 100% FREE to Sign Up for our Email Newsletter!
Disclaimer: This website provides information about cryptocurrency and related stock market investments. This website does not provide investment advice and should not be used as a replacement for investment advice from a qualified professional. This website is for educational and informational purposes only. The owner of this website is not a registered investment advisor and does not offer investment advice. You, the reader, bear responsibility for your own investment decisions and should seek the advice of a qualified securities professional before making any investment. This Feature Article & YouTube Video is SPONSORED CONTENT. We are expecting to be compensated as much as $10K USD via bank wire by a third party for the advertising / marketing of this publicly traded company. Please read our Full Disclaimer: https://dexwirenews.com/disclaimer/